A chloride of silicon contains 79.1 mass cl – A chloride of silicon containing 79.1 mass % Cl is a versatile compound with significant industrial applications. Its unique chemical composition and properties make it an essential component in the production of various chemicals and materials.
This chloride of silicon, commonly known as silicon tetrachloride (SiCl4), is a colorless liquid at room temperature with a pungent odor. It is highly reactive and reacts readily with water, releasing hydrogen chloride gas. Due to its reactivity, SiCl4 must be handled with proper safety precautions.
Chloride of Silicon

A chloride of silicon is a compound containing silicon and chlorine atoms. The chemical formula of a chloride of silicon that contains 79.1 mass % Cl is SiCl 4. This formula indicates that the compound contains one silicon atom and four chlorine atoms.
The mass percentage of Cl in SiCl 4can be calculated using the following formula:
“`Mass % Cl = (Mass of Cl / Mass of SiCl 4) x 100%“`
Substituting the atomic masses of Si (28.09 g/mol) and Cl (35.45 g/mol) into the formula, we get:
“`Mass % Cl = (4 x 35.45 g/mol / (28.09 g/mol + 4 x 35.45 g/mol)) x 100% = 79.1%“`
Physical and Chemical Properties: A Chloride Of Silicon Contains 79.1 Mass Cl

Physical Properties
Silicon tetrachloride is a colorless liquid at room temperature. It has a density of 1.52 g/cm 3and a melting point of -70 °C.
Chemical Properties
Silicon tetrachloride is a reactive compound. It reacts with water to form hydrochloric acid and silicic acid:
“`SiCl 4+ 2H 2O → 4HCl + H 2SiO 3“`
It also reacts with other substances, such as metals and bases.
Production and Applications
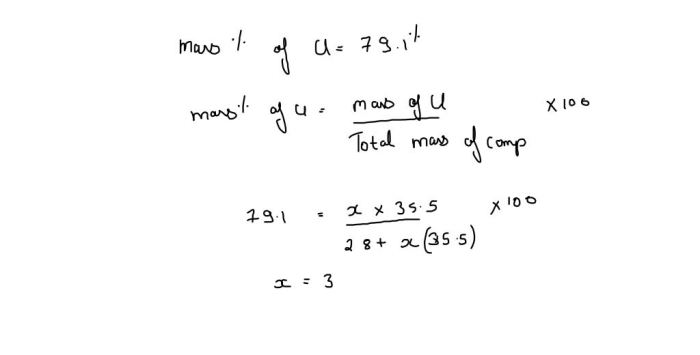
Production
Silicon tetrachloride is produced industrially by the reaction of silicon with chlorine gas:
“`Si + 2Cl 2→ SiCl 4“`
Applications, A chloride of silicon contains 79.1 mass cl
Silicon tetrachloride is used in the production of other chemicals, such as silicones and glass. It is also used as a catalyst in some chemical reactions.
Safety Considerations
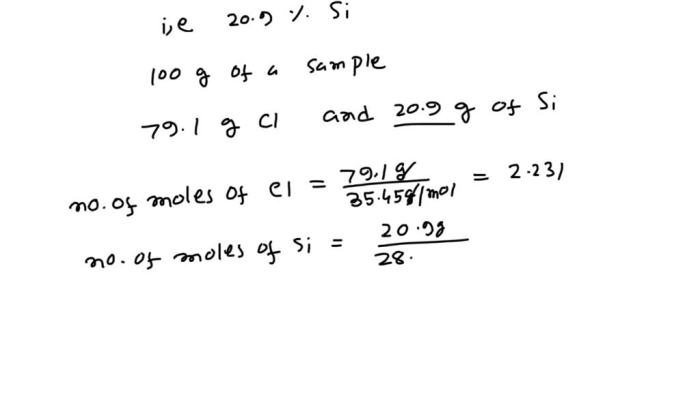
Silicon tetrachloride is a corrosive and toxic compound. It can cause skin and eye irritation, and inhalation of its vapors can cause respiratory problems.
Appropriate safety measures should be taken when handling and using silicon tetrachloride. These measures include wearing protective clothing, gloves, and eye protection, and working in a well-ventilated area.
Frequently Asked Questions
What is the chemical formula of a chloride of silicon containing 79.1 mass % Cl?
The chemical formula is SiCl4.
What are the physical properties of a chloride of silicon containing 79.1 mass % Cl?
It is a colorless liquid at room temperature with a pungent odor and a density of 1.52 g/cm3.
What are the chemical properties of a chloride of silicon containing 79.1 mass % Cl?
It is highly reactive and reacts readily with water, releasing hydrogen chloride gas.
What are the applications of a chloride of silicon containing 79.1 mass % Cl?
It is used in the production of semiconductors, glass fibers, and other silicon-based materials.